AgPlus are excited to share our initial success of Covid-19 antibody assay development.
“This is the most serious public health emergency in a hundred years”
Matt Hancock (May 2020)
 As of 2nd July 2020, the SARS-CoV2 pandemic had topped 10 Million cases worldwide and claimed over half a million lives. The immune system of the global community could not fight this new pathogen which has proven to be highly infectious and extremely deadly. Rapid point of care tests can make a significant contribution to reducing the spread of COVID-19 in several ways:
As of 2nd July 2020, the SARS-CoV2 pandemic had topped 10 Million cases worldwide and claimed over half a million lives. The immune system of the global community could not fight this new pathogen which has proven to be highly infectious and extremely deadly. Rapid point of care tests can make a significant contribution to reducing the spread of COVID-19 in several ways:
- Detection of the presence of the virus in individuals
- Monitoring of the population for antibodies in the bloodstream to determine the percentage of the population exposed.
- Supporting drug and vaccine development.
As the pandemic continues and the understanding of COVID-19 improves, regulators have placed greater emphasis on efficacy of tests, particularly in field, to ensure they can be deployed with confidence. Widespread testing of SARS-CoV2 virus is critical to know when and if people can return to their normal lives and a rapid point-of-care antigen test would support this. Antibody testing helps to determine whether the individual was ever infected - even if the person never showed symptoms. These tests support monitoring and responding to the COVID-19 pandemic.
AgPlus are proud to announce
we are developing two assays to support the COVID-19 diagnostic effort.
AgPlus has been working on an antigen assay and antibody assay. We have successfully secured grants from Innovate UK and DASA to support the work. Recognising the need to respond quickly we have utilised our proven lab-based electrochemistry technology, originally developed at the National Physical Laboratory and adapted this as a desk top solution that does not require a sterile environment, sophisticated equipment or extensive training.
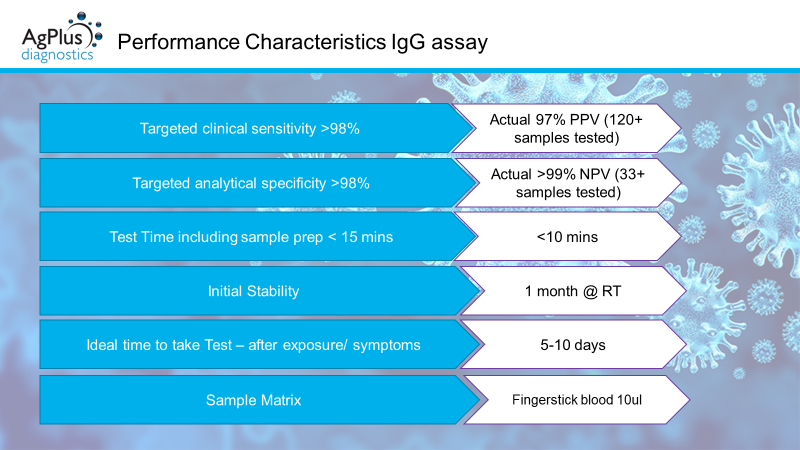
We have completed some initial clinical testing of our Antibody assay utilising over 30+ normal (pre-COVID samples), including samples positive for CMV IgG and around 120+ COVID-19 positive samples as confirmed by PCR. This testing has determined the assay has specificity of >99% and a sensitivity of 97%. The assay will now be further optimised and tested in field to ensure efficacy, prior to submitting to the MHRA. Working closely with our partner Marble Product Design we are upgrading our bench top readers with an interface and GUI to ensure ease of use at point of testing.
Our antigen assay is in earlier development and after some initial feasibility testing, we are now planning a contrived clinical study to determine limit of detection following on with an in-field study to determine sensitivity and specificity. This information will be reported in subsequent updates.
We are always looking for potential business to business partners. If you would like further information on AgPlus or any of our assays, please do get in touch.